We know that over half of us use supplements in one form or another. In the US alone there are more than 85,000 different supplement products on the market and the global supplement industry is worth $115 billion. One of the hottest segments is CBD/cannabidiol nutraceuticals, valued in the US alone at $3.8 billion and still forecast to increase. Wow. Since supplement industry regulations don’t adequately protect consumers, we all have to take ownership of understanding how to make wise decisions in our supplement choices.
In What Circumstances Can Supplements Be Helpful?
Our approach: Food first, supplement next as needed
In clinic we are always working with patients to help support optimal dietary intake and healthful therapeutic or maintenance eating patterns – we conduct regular Nutrient Intake Analyses for that very purpose. In our experience, we can’t out-supplement a poor diet.
However, there are times when it isn’t possible to get the right nutrient quantities through food, when we might need to move to a higher therapeutic dosing, or we need to lean on non-nutrient nutraceuticals (like botanicals, enzymes, free form amino acids, fibers, probiotics, molecular substrates, glandulars, or distilled bioactive compounds) to support the healing process and optimize health.
That’s when we use dietary supplements and when want to know we’re choosing high quality, safe supplements that do what they’re supposed to. If you’re choosing supplements for yourself, here is how to think like a nutritionist…
First, Understand How Supplements Are Regulated
Let’s review of the regulatory environment of supplements. (Tip – if you already agree that the supplement industry regulations don’t protect consumers adequately, you can skip down to the next section to find out what to do).
The US Food and Drug Administration (FDA) has been involved with the regulation of supplements in varying capacities since 1906. This flow chart explains the outline of how regulations have evolved over the last 120 years.
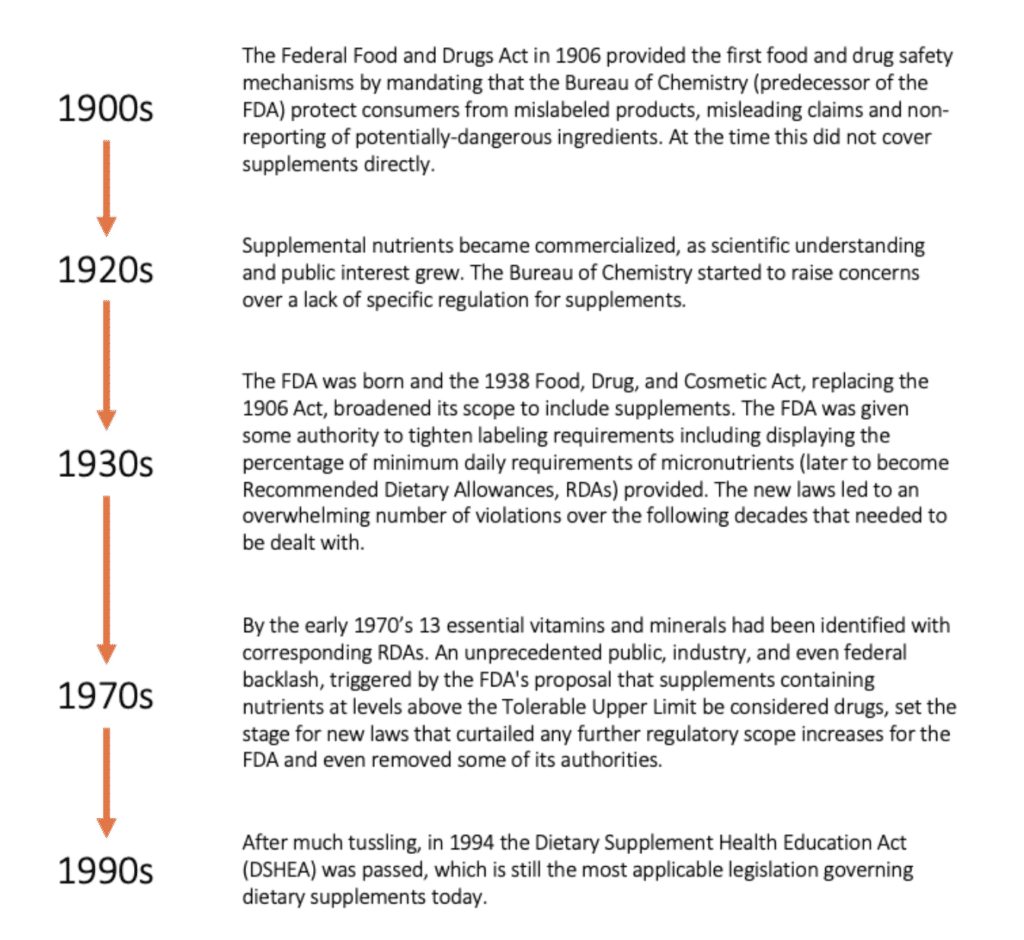
DSHEA regulations, and the FDA structure and funding, do present some ongoing challenges. Here are three major concerns:
- Quality Standards are Basic and Not Always Complied With. Current Good Manufacturing Processes (cGMPs) are quality standards mandated to dietary supplement companies by the FDA. However, these guidelines are basic, written to be flexible, and often don’t provide enough guidance for implementation. In addition, the FDA resources only stretch to monitoring 5-10 percent of supplement firms in any calendar year, and it is thought that over half of firms inspected by the FDA are not fully compliant with cGMPs.
- Supplements are not required to gain premarket approval (for safety, purity, quality) from the FDA. New-to-market ingredients can be determined as Generally Recognized as Safe (GRAS) by the producer, and FDA notification of new GRAS ingredients is voluntary for supplements. This loophole places the burden on the FDA to prove a product or ingredient is unsafe. This is a time consuming and costly process for the FDA making it difficult to remove products from the market over safety concerns. Ephedra, for example, was removed from the market in 2002 after a challenging process that took eight years and involved 10,326 poisoning cases, 108 critical-care hospitalizations, and seven deaths.
- Adverse Event Reporting is Lacking. The problem with adverse events reporting for dietary supplements is that most are reported to US poison control centers (PCCs). Here they are labeled as poisonings. The lack of communication between the FDA’s Adverse Event Reporting System (CAERS) and PCCs means the FDA’s ability to determine public health threats is compromised.
Now, let’s look at what we should all be doing to ensure we choose high quality, safe supplements:
1: Vet Labels
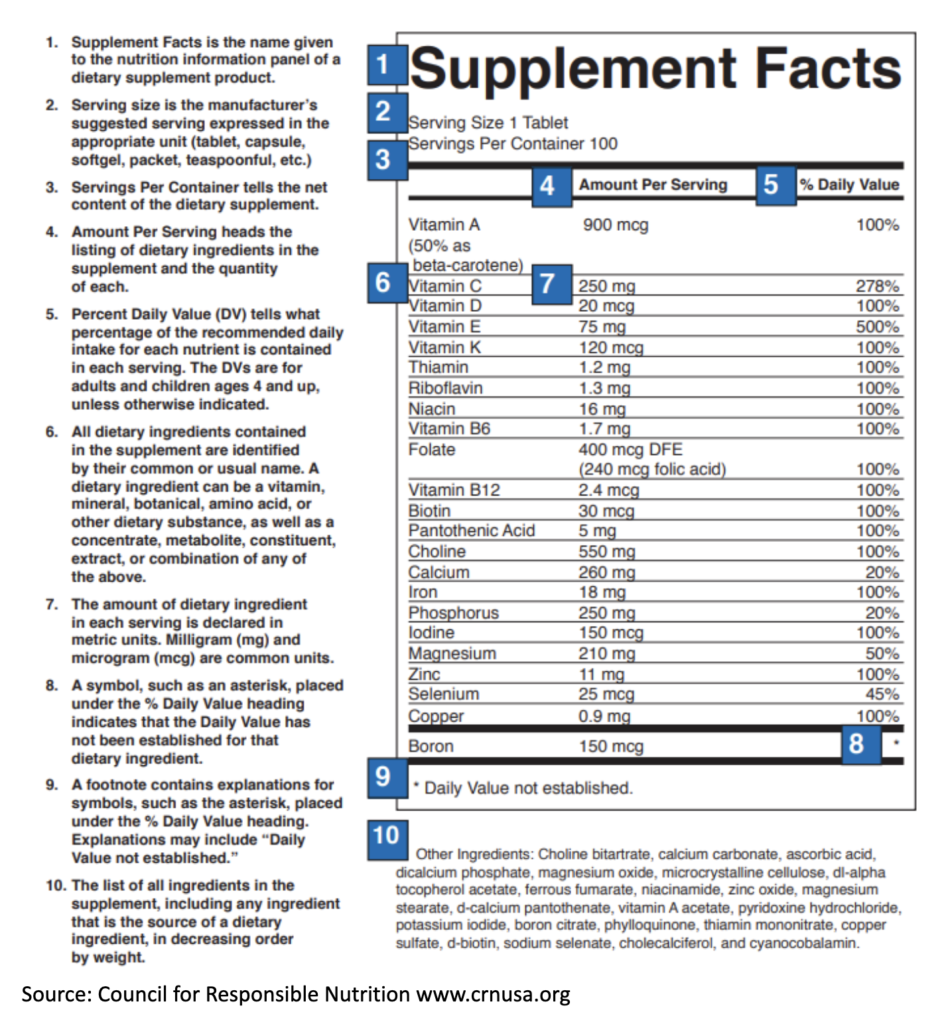
Understanding Supplement Facts
Just like food products, supplements are required to have a standardized label called “Supplement Facts.” On this label you will see a breakout of vitamins and minerals along with the Amount Per Serving and Percent Daily Value. We like this graphic from the Council for Responsible Nutrition that explains how to read this box.
Nutrient form
There are numerous forms each vitamin and mineral can take in a supplement. Your selection should take into account purpose, bioavailability, bioactivity, and synthetic/natural formulations. Here’s more about each of those aspects, with examples:
- Purpose: What do you want to use the supplement for? Take magnesium as an example – For most people, magnesium oxide stays predominantly in the GI tract and will help get your bowels moving. Magnesium citrate is a good all-rounder for absorption with a gentle laxative effect. Magnesium glycinate won’t impact your bowels to the same extent and has a calming, sleep-balancing effect. Folate types are another example – Folinic acid, a form of folate is often used specifically for brain support in specific situations since it more readily crosses the blood-brain barrier.
- Bioavailability: How much of that nutrient is available to be absorbed from the intestines and utilized? This is important if we want the effect to occur outside of the intestines only. Calcium citrate, for instance, is recommended over calcium carbonate for those with low stomach acid or taking acid-blocking medications since its bioavailability is maintained even if stomach acid is low. Other forms of calcium need stomach acid to be released and absorbed and have low bioavailability when stomach acid is also low.
- Active forms: Another consideration is a bioactive vs nonbioactive form of a nutrient. Supplemental vitamin B6 is commonly available as pyridoxine. However, the pyridoxal-5’-phosphate (P5P) form is a match for the active form that the body uses and may be more helpful in some situations. Similarly, 5-methyl folate or folinic acid are considered more bioactive forms of folate than tetrahydrofolate.
- Synthetic vs natural: Synthetic forms of nutrients are often used instead of natural forms. Folic acid, the synthetic form of folate, is commonly found in less expensive supplements and is used to fortify foods. It is less easy for the body to utilize and for some individuals may even interfere with proper folate metabolism. Natural ingredients usually list food sources on the label.
Inactive ingredients
This is where we look at what else is in the dietary supplement, in addition to the ingredients you’re buying it for. Additional ingredients are sometimes included to enhance the delivery of the nutrient to where it needs to go in the body, or its activity – like piperine in curcumin supplements. But sometimes there are ingredients like fillers, additives, and artificial flavorings and colors that are included for non-health reasons such as palatability, anti-caking, bulk, and shelf stability. Potentially-harmful ingredients include artificial colors, magnesium silicate (talc), and titanium dioxide. Some ingredients may not be suitable for vegetarians or those with allergies.
2: Seek Independent Confirmation of Authenticity
So you’ve checked it out and the label looks good. What next? There are several other aspects of quality to consider that you can’t assess for just by looking at the label:
- Identity, strength, purity, and composition: Supplement manufacturers usually source their individual ingredients from third party suppliers. cGMPs require testing to be done to confirm the identity, strength, purity, and composition of the ingredient in question. Manufacturers are not allowed to rely on the raw material supplier’s certificate of analysis, and must carry out independent authentication (although, as noted above, this doesn’t always happen).
- Additional contaminants: Possible contaminants that are not listed on labels include solvents, bacteria, yeast, mold, melamine, heavy metals, agricultural chemicals, aflatoxin, dioxins, PCBs, and even pharmaceuticals. These have all been found in supplements that are readily available from certain retailers and online. Sildenafil (Viagra), for example, has been found in sexual enhancement supplements. Heavy metals have been found in traditional remedy supplements.
- Rancidity: Supplements such as delicate omega-3 fatty acids can become rancid if not adequately protected. Rather than provide the cardiovascular protection that you may be looking for in an omega-3 fatty acid supplement, rancid omega-3s can actually raise LDL cholesterol. Supplement suppliers should ensure vulnerable products do not turn rancid through appropriate expiry dates, storage conditions, and stabilizing ingredients.
- Potency: Not all brands test their end products for potency, which would confirm that each dose contains what its label claims. GMPs don’t state that manufacturers need to do finished product testing. The best quality supplement providers do go above and beyond cGMP guidelines, including performing end-product testing.
Because the FDA does not perform widespread checks, we also like to look for evidence of ongoing third-party verification. This might include verification from organizations such as ConsumerLab.com, NSF International and the US Pharmacopeial Convention, which test that the product meets each organization’s respective criteria. But universal standards are lacking. You can read more about these certification programs here.
Another form of verification to ask for is a supplement’s Certificate of Analysis. Ideally, the analysis is performed by an independent, third-party laboratory. Even then, we should keep our wits about us – there are reported instances of incorrectly-reported results, errors in which product was reported on, and even occasional fabrication of results.
Red flag example! Just recently one of our nutritionists, Jill Sheppard Davenport, reached out to a popular consumer protein powder brand for a certificate of analysis on their product. Something we routinely do. This particular vendor said they never give out that information. This is a red flag. A company should readily provide you with recent and ideally, third-party, testing results, in a reasonable time frame (we understand this might not always be immediately). Consider lack of transparency as a warning sign.
Beware of just plain fakes:
You may have heard of brand knock-offs being sold on Amazon.com – last year, a startling Wall Street Journal report found over 4,000 items for sale on Amazon.com that had been declared unsafe, were deceptively labeled or were banned by federal regulators. Nutritional supplements, such as probiotics and pet vitamins can be among the fakes you may find. We’ve had our own patients report that when they purchased product refills from Amazon.com they found the capsules looked and smelled different – highly suspect. Any kind of nutritional supplement from Amazon third-party sellers should be well vetted. The WSJ even followed up their report with an exposé of just how easy it was for them to set up a test account selling items they pulled, literally, from dumpsters.
3: Perform Final Checks
So you’ve gone through all this trouble of vetting a specific supplement and it’s jumped through all your hoops… that means it should be safe, right? Not necessarily. Here are some final things to think about:
Check for interactions:
Dietary supplements may be available without a prescription, but that doesn’t mean anyone should take them. Even small doses of nutrients, botanicals, and other compounds can have profound effects at a cellular level. This is especially true for people who are already taking prescription drugs. For example, if you take the drug Sertraline, also known as Zoloft, for depression you should not take the supplements 5-HTP, L-Tryptophan, and St. John’s Wort unless supervised by a prescribing physician. There is a potential for a negative interaction resembling side effects of sertraline. Drugs.com has an interactions checker that you can reference. Fullscript also has a handy quick reference guide for some common nutrient interactions.
Check for potential toxicity:
In addition to drug-supplement interactions, we need to consider the issue of toxicity. Liver injury is of concern especially in combination products marketed for weight loss. Consumer Reports publishes a useful list of currently-used and potentially harmful supplement ingredients to watch out for here. For practitioners, we recommend Natural Medicines Database as a comprehensive guide to toxicity potential (as well as the latest research on efficacy and use, nutrient depletions, and interaction checkers). We also prefer glass supplement containers where possible, to further reduce exposure to endocrine-disrupting chemicals from plastics.
A note about packaging toxicity: The evolution of dietary supplement packaging towards lower ecological impact, and reduced use of plastics with endocrine-disrupting chemicals, has been slow. We hope that this will change in the near future! For the moment, choose glass containers where they are available, and ask your supplement providers to work on this issue.
Check for allergens:
If you know you have reactions to certain foods or ingredients, you must check for and avoid them. Commonly used potential allergens are corn starch (contains corn) and lactose (typically from dairy sources). If you are someone that must strictly avoid gluten, you must look for supplements that are labeled “gluten free.” Don’t be afraid to contact the supplement company if you are unsure. Make sure you are happy that they test their products to contain less than 20 parts per million of gluten.
Check your cumulative dosing:
More of something good is not always better. The Institute of Medicine publishes Tolerable Upper Intake Levels (UL), which are the maximum daily intake unlikely to cause adverse health effects. This upper limit doesn’t just apply to supplements – it also includes nutrient content from the food you eat, both natural and fortified. Looking at dosing cumulatively, there are easily instances where we can exceed the UL. Take folate as an example: It is not uncommon for someone to exceed the UL of 1.0 mg/d of folate if they are eating fortified foods such as cereal and bread, along with taking a multivitamin supplement.
The Bottom Line
Nutritional supplements tremendous tools to help achieve optimal health. Yet we have to be careful about which supplements we choose to use. We hope this information will encourage you to do your homework when choosing supplements or reach out to a qualified professional who can help you.
In our clinic, we only work with professional-grade supplement companies that we have extensively vetted and trust. That also goes for the supplement companies that we showcase on this site and who help fund (but not determine) our content. We work as a team to carefully examine any new-to-market supplements and decide whether they are right for us to recommend.
Resources to use
These are a handful of our favorite resources for information on dietary supplements:
- Consumer Lab (individual product evaluation, recalls, free newsletter but access to most of the site requires a membership): https://www.consumerlab.com/
- Interaction checkers: https://www.drugs.com , https://reference.medscape.com/drug-interactionchecker, https://naturalmedicines.therapeuticresearch.com/
- Linus Pauling Institute (summaries of research and micronutrients along with a free newsletter): https://lpi.oregonstate.edu
- Where to submit adverse event reports? CAERS Safety Reporting Portal: https://www.safetyreporting.hhs.gov/SRP2/en/Home.aspx?sid=5221c5ae-63c6-47dc-b402-c8f22e9266d7
- Dietary Supplement Fact Sheet – National Institutes of Health Office of Dietary Supplements https://ods.od.nih.gov/factsheets/list-all/
- Dietary Supplements 101 – Fullscript: https://s3.amazonaws.com/fs-marketing-files/hcp/Supplements101-Web.pdf
A shout out to our Nutrition Residents, Gretchen DePalma and Karin Michalk, who contributed to this article:
Gretchen DePalma is a Certified Nutrition Specialist (CNS) candidate and certified Health Coach. She holds a Master of Science degree in Nutrition and Integrative Health from Maryland University of Integrative Health and a Bachelor of Science in Finance from Wake Forest University. She has developed a passion for optimizing women’s health and fertility. In a time when chronic lifestyle diseases are so prevalent, especially among children, she feels it is critical that women have support to properly nourish and care for their bodies prior to conceiving. Prior to pursuing a career in nutrition, she worked in investment banking and strategic planning.
Karin Michalk is a Physical Therapist and has recently completed a Masters in Human Nutrition and Functional Medicine from The University of Western States, Portland, OR. Through her work with patients across the lifespan she has come to realize the fundamental importance nutrition plays in keeping and regaining health. She is working towards obtaining the CNS credential and is a member of the Functional Nutrition Residency Program. Very soon, Karin will be bringing a blend of personalized nutrition and PT services to her clients so they may find their way back to optimal health.
This article originally appeared on www.drkarafitzgerald.com.


